

904 mol PBI2 23 408g KClO3 24 748g CO2 25 4.48g H2 26 83. Theoretical mole ratio of NaHCO3 to NaCl 1: NaHCO3(s) + 2HCl(g) -> 2NaCl(g) + H2O(l) + CO2(g) Molar Ratio 1:2 -> 2: Conclusion: Using data from this experiment, we were able to determine the experimental mole-to-mole ratio between sodium bicarbonate and sodium chloride. 70 g If the theoretical yield for a chemical reaction is 100g and the percentage yield is 70% the actual yield is. excess reactant The substance that is not completely used up in a chemical reaction is known as the. actual yield The measured amount of product obtained from a chemical reaction is called the. limiting reactant The substance that restricts the amount of other reactant used in a chemical reaction is known as the. Unknown: actual yield/theoretical yield * 100 Percentage yield = moles of a, moles of b grams of a > _ > _ > grams of b 1 mol C7H6O3/138.13 g C7H6O3 The conversion factor for deriving the number of moles of salicylic acid, C7H6O3 from a given number of grams of salicylic acid is. Given: S A chemist must determine how many grams of sulfur are required to produce 800 g of sulfur dioxide. You should have found that the reaction could form 0.01750moles of alum ( Help Me ). Now convert moles of Al to moles of alum using the stoichiometric factor from the balanced chemical equation. You should get 0.01750 moles of Al ( Help Me ). Unknown: 800 g SO2 A chemist must determine how many grams of sulfur are required to produce 800 g of sulfur dioxide. First, we must determine the moles of Al in 0.475 g of Al. Given: O2 A chemist must determine how many moles of oxygen can be produced from 50 mol of water.
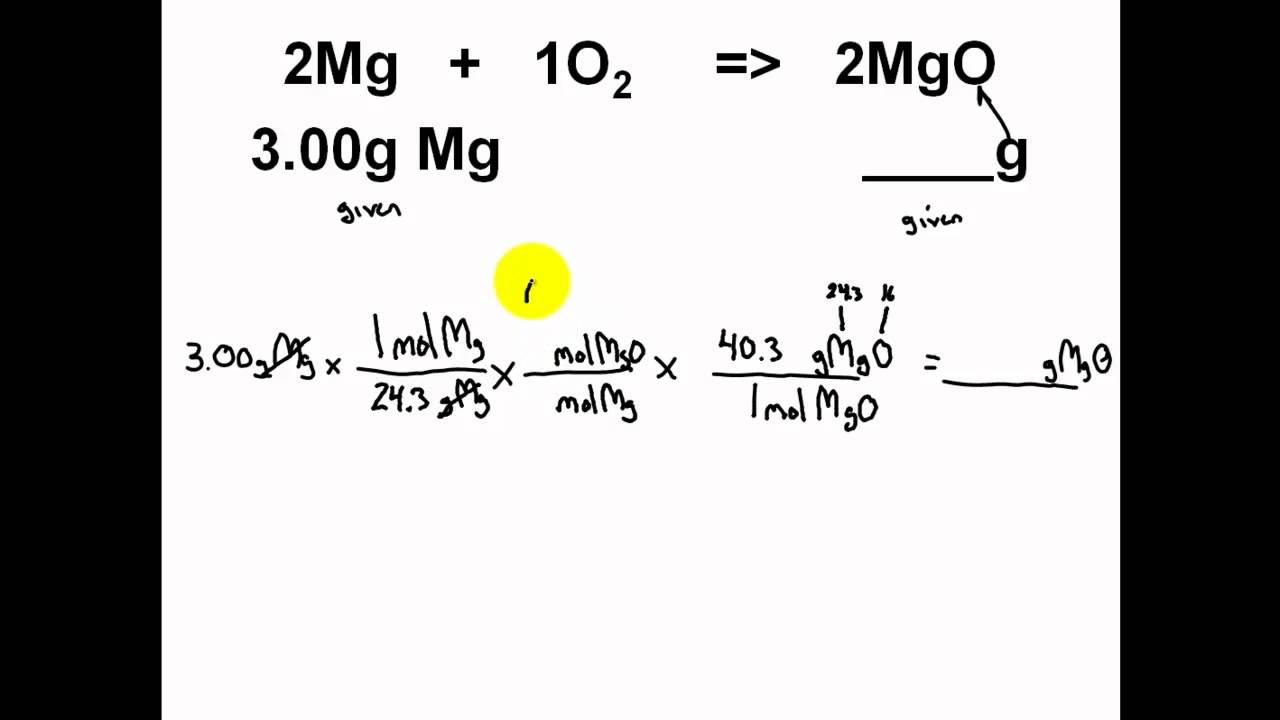
If you wanted to use up the entire 32.2 mol supply of H2, you would need 1/2 of the 32.2 mols for the required mols of CO, or 16.1 mols of CO. Formula with the smallest whole number mole ratio of the elements for a. So 12.7 mols of CO would require twice the amount of mols of H2, or 25.4 mols of H2. Chemistry (12th Edition) answers to Chapter 11 - Chemical Reactions - 11. In a similar manner, convert the final mass of collected sodium chloride product to moles (via its molar mass). You need twice as much H2 as CO since their stoichiometric ratio is 1:2. Unknown: 50 mol H2O A chemist must determine how many moles of oxygen can be produced from 50 mol of water. Analysis: Experimental Mole-to-Mole Ratios Convert the initial mass of sodium bicarbonate (or carbonate) reactant to moles (via its molar mass). Given: CO A chemist must determine how many grams of carbon monoxide are needed to produce 20 mol of CO2 in the reaction. Unknown 20 mol CO2 A chemist must determine how many grams of carbon monoxide are needed to produce 20 mol of CO2 in the reaction. H2 A chemist must determine how many moles of H2 can be produce from 500 g of H2O in the reaction. 500 g H2O A chemist must determine how many moles of H2 can be produced from 500 g of H2O in the reaction.


 0 kommentar(er)
0 kommentar(er)
